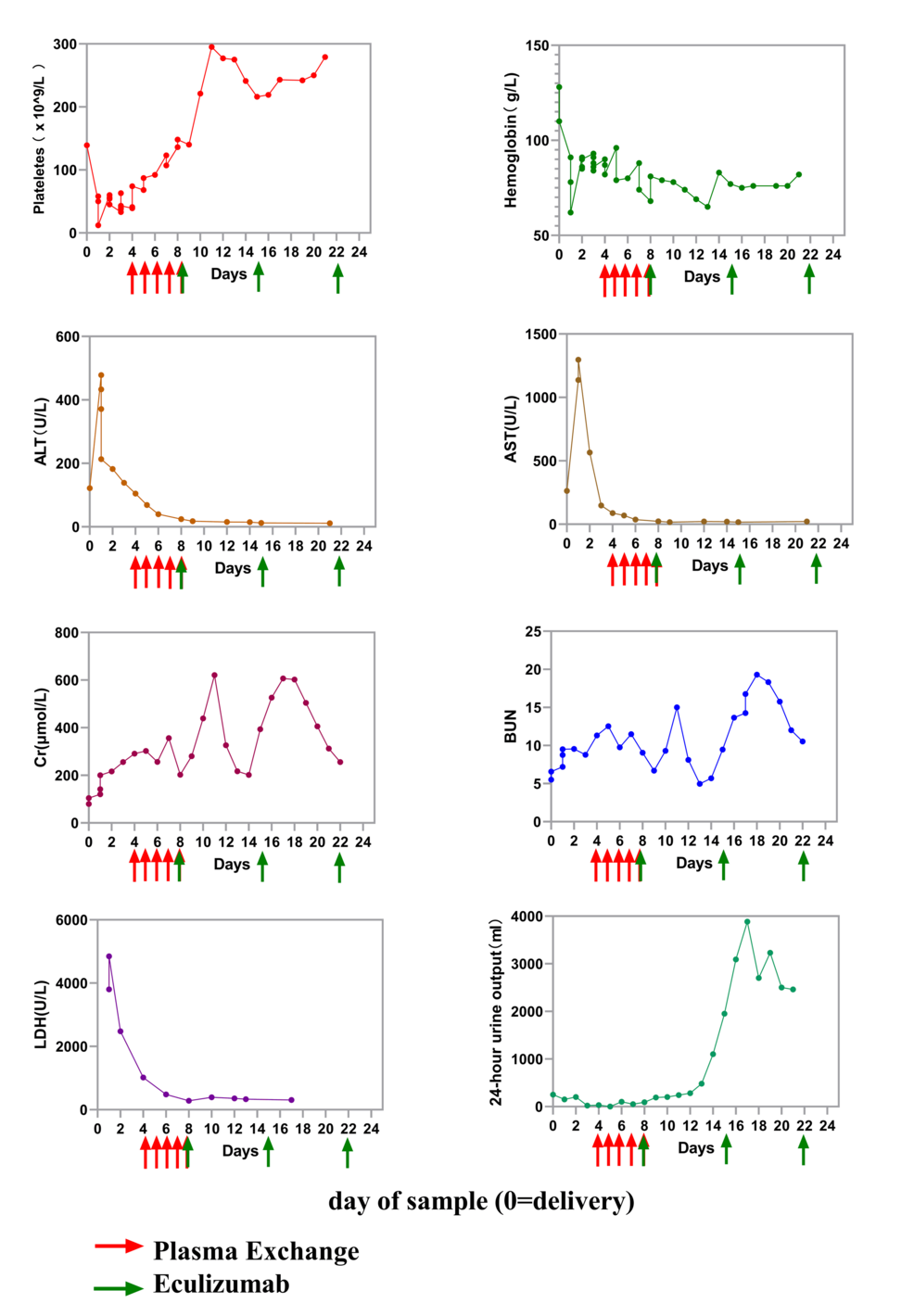
Birmingham Spin-Out Linear Diagnostics Secures £1 Million to Develop Rapid STI Testing Technology

Birmingham-based Linear Diagnostics, a pioneering spin-out company, has recently received a significant funding boost of £1 million to enhance the development of a cutting-edge rapid test aimed at detecting sexually transmitted infections (STIs). This initiative is in collaboration with the National Institute for Health and Care Research (NIHR) HealthTech Research Centre (HRC) in Diagnostic and Technology Evaluation and the North East Innovation Lab, which is a segment of Newcastle Hospitals.
The ambitious project focuses on creating a low-cost and highly accurate near-patient diagnostic platform. The objective is to diagnose infections more swiftly than any currently available commercial alternatives, addressing the urgent need for rapid STI testing amidst rising infection rates.
This funding, allocated through the NIHR's Invention for Innovation program, will support a comprehensive three-year project aimed at finalizing the test design and ultimately conducting clinical evaluations in real-world settings. This crucial phase will prepare the technology for clinical trials, marking a significant milestone in its development.
Linear Diagnostics is leveraging its innovative Exponential Amplification (EXPAR) technology. This breakthrough method allows for the rapid detection of bacterial DNA by amplifying the signal at unprecedented speeds. Initially developed and validated during the COVID-19 pandemic, this technology's efficacy was documented in the Proceedings of the National Academy of Sciences. Remarkably, the company has demonstrated that it can identify bacterial STIs, urinary tract infections, and even viral infections such as SARS-CoV-2 in as little as five minutes.
In light of the increasing prevalence of multi-drug-resistant strains of Neisseria gonorrhoeae, a bacterial STI, the focus on rapid testing has become even more critical. The emergence of these resistant strains has raised alarms within the medical community, necessitating swift identification and treatment to curtail the transmission of infections. Current testing methods, despite being user-friendly, often fall short of providing results within the target timeframe of 20 minutes.
The newly awarded funding will enable Linear Diagnostics to finalize the design of their testing cartridges and associated reader devices, paving the way for validation of the platform. Dr. Jean-Louis Duprey, Head of Research and Development at Linear Diagnostics, emphasized, “The most difficult criteria to achieve in diagnostic testing is combining rapidity with accuracy. While rapid lateral flow tests meet the ideal timeframe of 20 minutes for diagnosis, they often lack the necessary sensitivity and specificity. Conversely, Nucleic Acid Amplification Tests, although highly accurate, require laboratory analysis, resulting in a wait that can extend for days. Our goal is to create a near-patient device that resolves this dilemma.”
The NIHR HRC, hosted by the Newcastle upon Tyne Hospitals NHS Foundation Trust in collaboration with Newcastle University, will play a pivotal role in evaluating this pioneering technology. Dr. Jana Suklan, a Senior Methodologist at the HRC, expressed enthusiasm about the collaboration, stating, “The NIHR HRC in Diagnostic and Technology Evaluation is delighted to collaborate with the North East Innovation Lab to support Linear Diagnostics with their exciting technology. We are committed to thoroughly reviewing clinical guidelines and engaging with healthcare professionals, patients, and the public to ensure that the platform is optimally developed to improve patient care.”
Dr. Suklan further explained, “Our research will focus on identifying unmet needs, assessing current practices, and determining the ideal stage in the patient care pathway for implementing this technology. We will also evaluate the diagnostic accuracy of the test by statistically analyzing data collected by the innovation lab and ascertain whether adopting this technology represents a cost-effective solution for the NHS through health economic modeling. Importantly, our public contributors will guide this research to ensure it aligns with the needs of patients, the public, and caregivers.”
John Tyson, Head of the North East Innovation Lab, highlighted the significance of this collaboration, stating, “We are thrilled to continue our collaborative efforts with our partner innovators to support the development and evaluation of this groundbreaking test. By providing access to a wide array of clinical samples and NHS lab performance testing, we can establish the evidence required to propel new innovative technologies to the next developmental stage or facilitate their entry into mainstream medical usage.”