Unraveling the Complex Interplay Between CMK-1 and Calcineurin Signaling in Thermo-Nociception
In an intriguing exploration of cellular signaling pathways, researchers have conducted a systematic analysis of the interplay between CMK-1 and calcineurin signaling, uncovering a multifaceted regulatory network. This intricate relationship primarily reveals that these two pathways often act in opposition to each other, playing crucial roles in the regulation of thermal nociceptive adaptation.
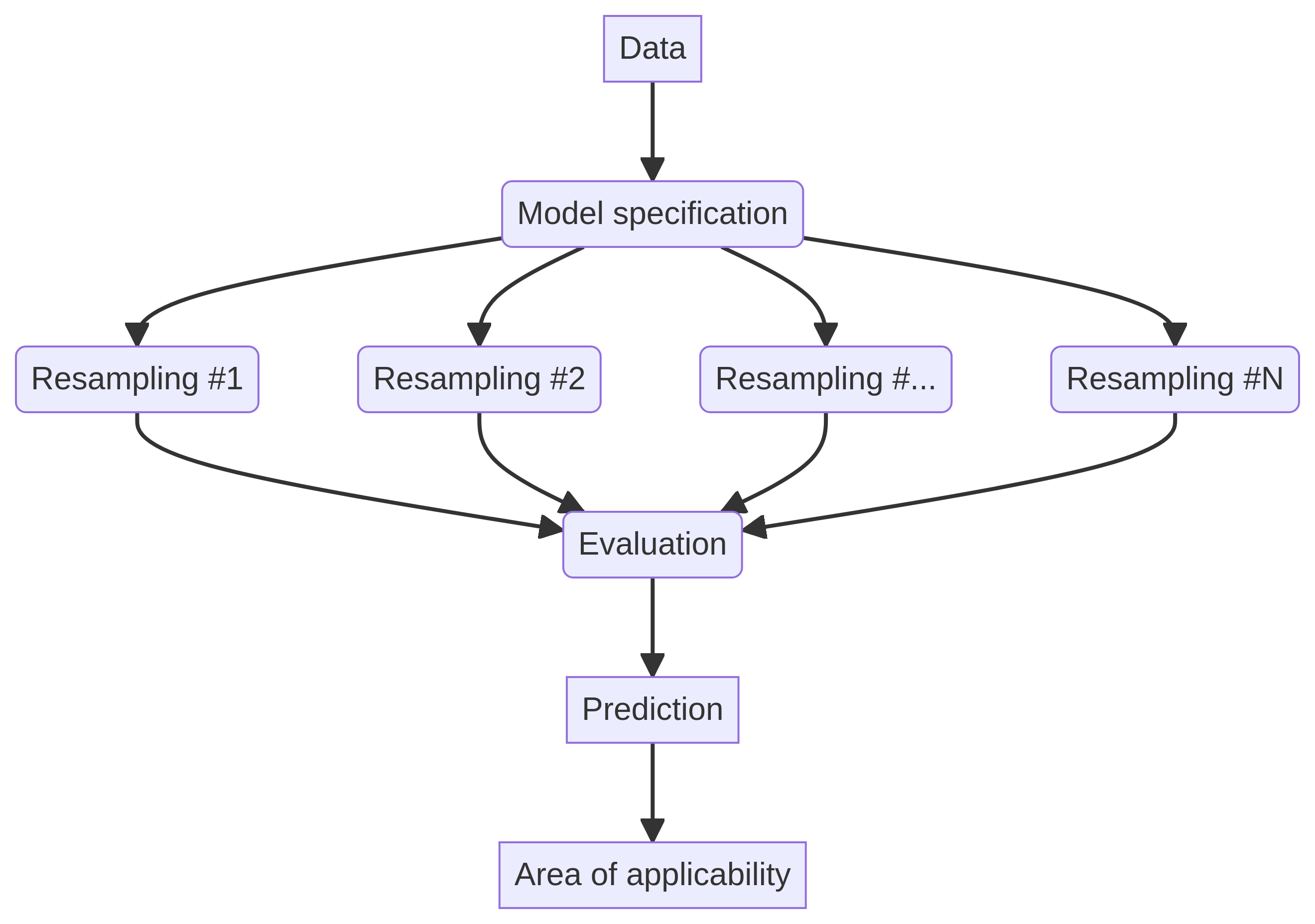
Notably, despite the concurrent inhibition of both CMK-1 and TAX-6/CnA, adaptation still occurs. This observation has led scientists to model their interactions as distinct regulatory events that govern a separate adaptation process. For a visual representation, refer to the model depicted in Figure 3D. This complexity, while challenging, serves as the simplest framework that can cohesively explain all measured interactions, with detailed illustrations available in Figure 3figure supplement 1.
Within this regulatory network, CMK-1 influences thermal nociception through three distinct pathways. The first pathway illustrates that CMK-1 promotes adaptation by inhibiting TAX-6/CnA, which conversely functions to suppress adaptation. The subsequent two pathways demonstrate that CMK-1 can independently inhibit adaptation or promote it, with both pathways modulated by TAX-6/CnA activity. This intricate balance between CMK-1 and calcineurin signaling could represent a sophisticated method for achieving nuanced modulation of thermal perception, effectively integrating previous activities with additional sensory input.
Such a complex regulatory scheme is unlikely to occur within a single neuronal cell type, which is consistent with findings from cell-specific approaches that identified multiple cellular loci of action within the circuit responsible for temperature sensation and the execution of reversal responses.
Previously, CMK-1 was documented to function autonomously within FLP neurons to influence the responses to thermal stimuli following prolonged exposure to noxious heat, as noted in the study by Schild et al. in 2014. FLPs are classified as 'tonic' thermo-sensory neurons, meaning their activity consistently reflects current temperature changes, a vital aspect of thermoregulation.
In the current study, researchers discovered that AFDs, rather than FLPs, serve as the primary cellular locus for CMK-1-dependent plasticity when faced with repeated short-duration thermal stimuli. Interestingly, AFDs are predominantly 'phasic' thermo-sensory neurons that generate activity spikes in reaction to temperature alterations, making them well-suited for encoding rapid thermal shifts and modulating the heat-evoked reversal circuit.
Previous laser ablation studies of AFD demonstrated a reduced reversal response when exposed to targeted infrared laser beams. However, using diffuse thermal stimuli that impacted the whole organism, researchers found that neither the genetic ablation of AFD nor selective inhibition of neurotransmission affected the animals' ability to execute heat-evoked reversals. Rather intriguingly, AFD was found to be crucial in facilitating an experience-dependent decrease in heat-evoked reversal responses.
Furthermore, it has been established that CMK-1 mediates both short-term and long-term adaptation of AFD's intracellular calcium activity in response to thermal fluctuations within an innocuous temperature range of 1525C, as detailed in Yu et al. (2014). While long-term changes were associated with shifts in gene expression, the mechanisms driving short-term adaptation remain unclear. Current understanding of how CMK-1 orchestrates thermo-nociceptive adaptation in AFD is still evolving. Potential mechanisms could involve alterations in AFD thermo-sensitivity, neuronal excitability, calcium dynamics, or the modulation of neurotransmitter release.
Equally intriguing is the unknown downstream circuitry involved in this process. One possibility is that CMK-1 signaling within AFD affects reversal responses through its direct interneuron partners, AIZ and/or AIY, or through extra-synaptic communication mechanisms involving neuropeptides. Future investigations will be essential to elucidate the downstream processes activated in AFD and beyond, determining how they modulate the animal's responsiveness to harmful heat stimuli.
On the other side of this signaling equation, calcineurin signaling has been shown to operate at multiple cellular loci to modulate the responses to thermal noxious stimuli. Enhanced TAX-6 activity in FLP neurons was found sufficient to boost reversal responses in naive animals. Given that FLP activity is known to favor reversal responses, one could propose that calcineurin activity might enhance FLP function.
Conversely, overactive TAX-6 activity observed in RIM neurons, and to a lesser extent in AVA/AVD/AVE neurons, also increased reversal responses in naive animals, but demonstrated an enhanced response upon repeated exposure. This implies that the influence of calcineurin signaling in these neurons varies based on prior experiences. While AVA/AVD/AVE neurons are largely thought to promote reversal responses, RIM neuron activity may modulate reversal responses depending on contextual factors.
One particularly striking discovery was that cell-autonomous overactivation of TAX-6 in interneurons significantly obstructs adaptation, regardless of any manipulations to CMK-1 activity. A hypothetical explanation for this could suggest that TAX-6/CnA activity in worms operates within the post-synaptic region of interneurons, adjusting synaptic strength through its action on ion channels or neurotransmitter receptors, paralleling mechanisms observed in mammalian models of synaptic plasticity.